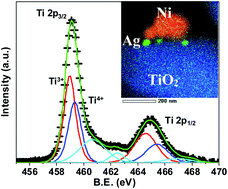
Synergistic effect of Ni–Ag–rutile TiO2 ternary nanocomposite for efficient visible-light-driven photocatalytic activity - RSC Advances (RSC Publishing)
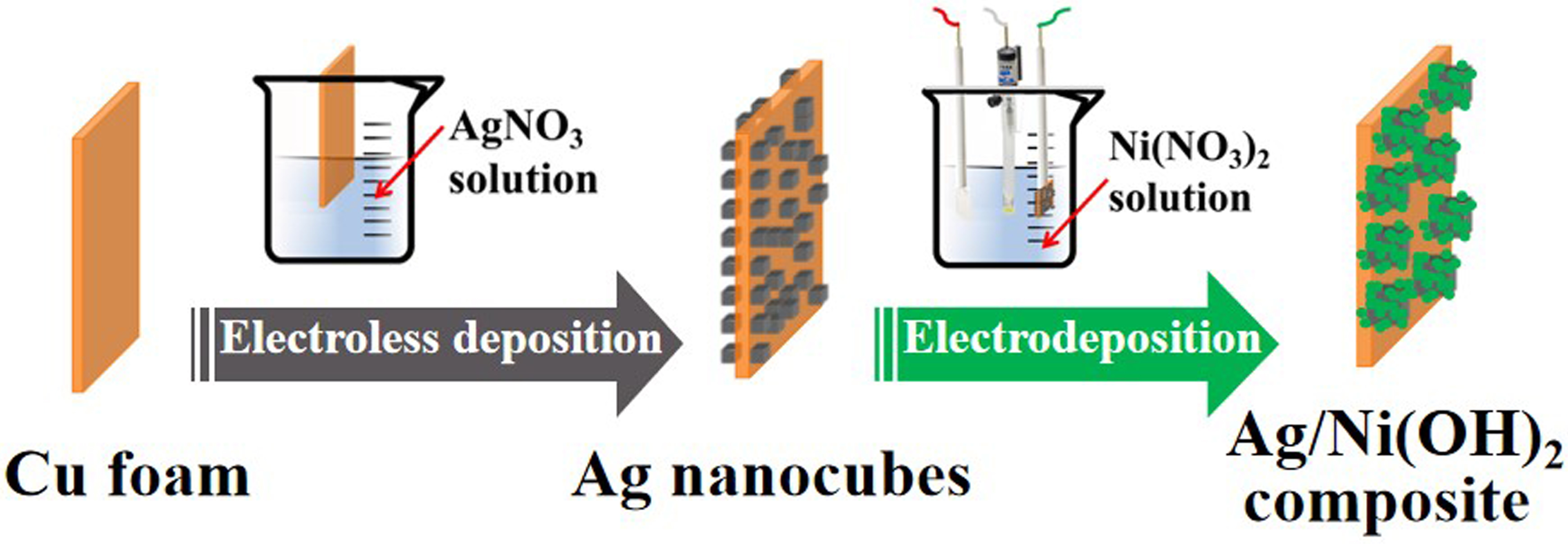
Metals | Free Full-Text | In Situ Construction of Ag/Ni(OH)2 Composite Electrode by Combining Electroless Deposition Technology with Electrodeposition
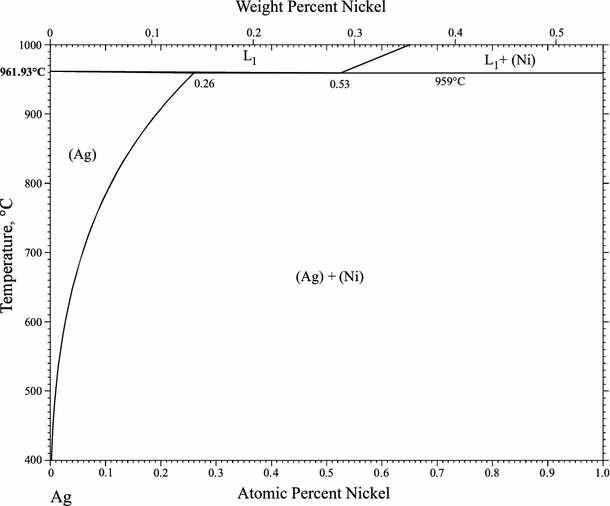
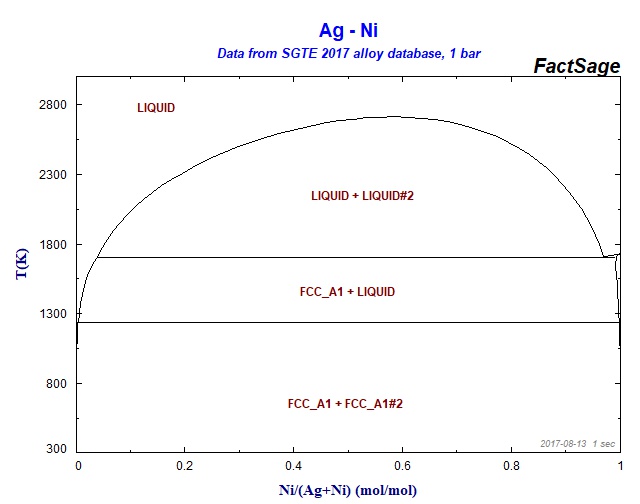
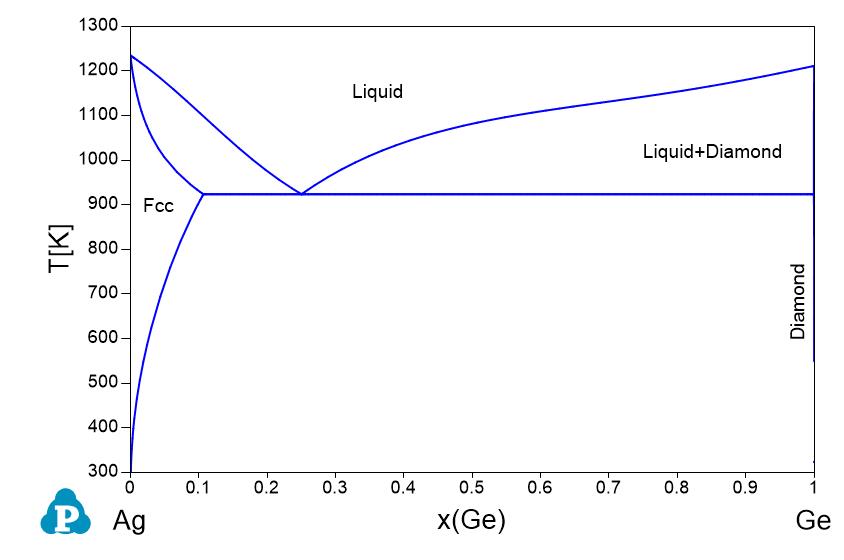
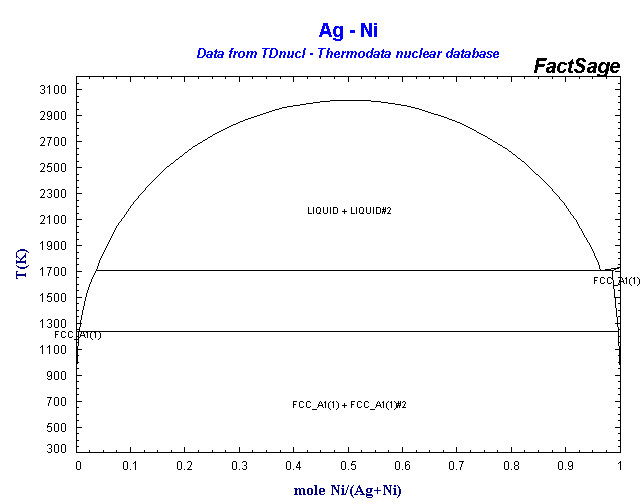
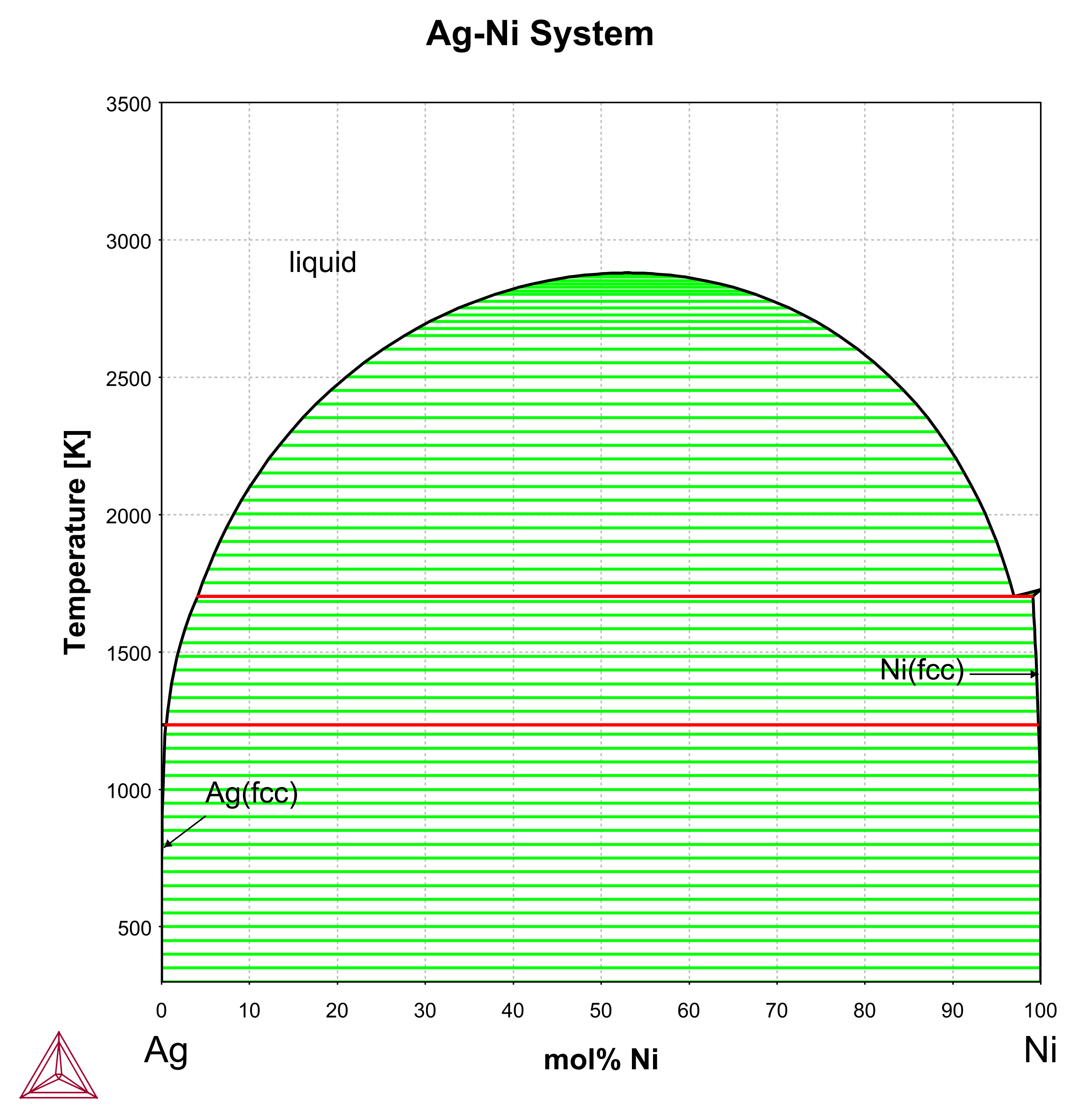
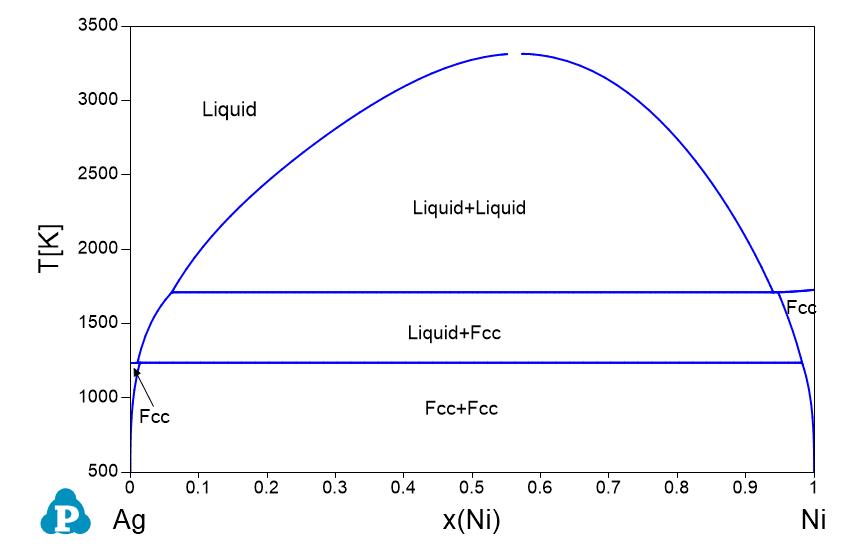
Supplemental Literature Review of Binary Phase Diagrams: Ag-Ni, Ag-Zr, Au-Bi, B-Ni, Co-Sb, Cu-Mn, Cu-Si, Cu-Zn, Fe-Zr, Li-Sb, Mg-Pu, and Si-Zr | SpringerLink

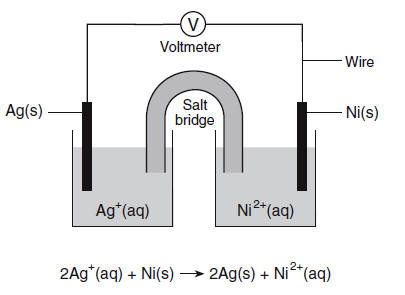
Derive Nernst equation for the cell. Ni(s) | Ni^2 + (aq. 0.1 M)||Ag^+ (aq. 0.1 M| Ag(s) and also find its cell potential. Given: E^∘Ag^+/ Ag = 0.80 volt and E^∘Ni^2 + /

Light reflectance of Ni/Ag contacts with different Ni thicknesses ( a )... | Download Scientific Diagram

UV-Vis spectra of pure Ag, pure Ni, Ag 75 Ni 25 (a), Ag 50 Ni 50 (b)... | Download Scientific Diagram

Electrochemical characteristics of silver/nickel oxide (Ag/Ni) for direct ammonia oxidation and nitrogen selectivity in paired electrode system - ScienceDirect

Derive Nernst equation for the cell. Ni(s) | Ni^2 + (aq. 0.1 M)||Ag^+ (aq. 0.1 M| Ag(s) and also find its cell potential. Given: E^∘Ag^+/ Ag = 0.80 volt and E^∘Ni^2 + /
For the cell reaction Ni(s) | Ni^2+(aq) || Ag^+(aq) | Ag(s) Calculate the equilibrium constant at 25°C. - Sarthaks eConnect | Largest Online Education Community

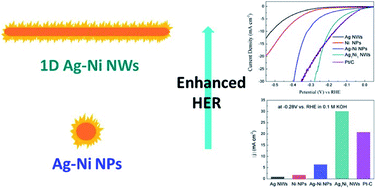
Synthesis of Ag–Ni–Fe–P Multielemental Nanoparticles as Bifunctional Oxygen Reduction/Evolution Reaction Electrocatalysts | ACS Nano